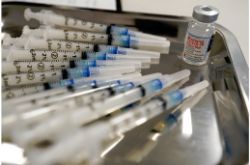
UNDATED (AP) — The U.S. Food and Drug Administration has authorized two changes to Moderna’s COVID-19 vaccine that can provide extra doses from each vial. The agency said late Thursday it approved new vials from Moderna that can contain up to 15 doses each, compared with the original vials designed to hold 10 doses. Additionally, regulators said providers can safely extract up to 11 doses from the original 10-dose vials. Those changes will be added to instructions for health care workers. The dosing updates should help bolster U.S. supplies and speed vaccinations as the U.S. nears 100 million inoculations against COVID-19.
Photo: FILE – This Thursday, March 18, 2021 file photo shows syringes filled with the Moderna COVID-19 vaccine at a pop up site in the Queens borough of New York. (AP Photo/Mary Altaffer)