CAMBRIDGE, Mass. (AP) — Moderna says new results show its COVID-19 vaccine works well, and it will seek emergency regulatory OK in U.S., Europe.
Photo: (AP Photo/Hans Pennink, File)
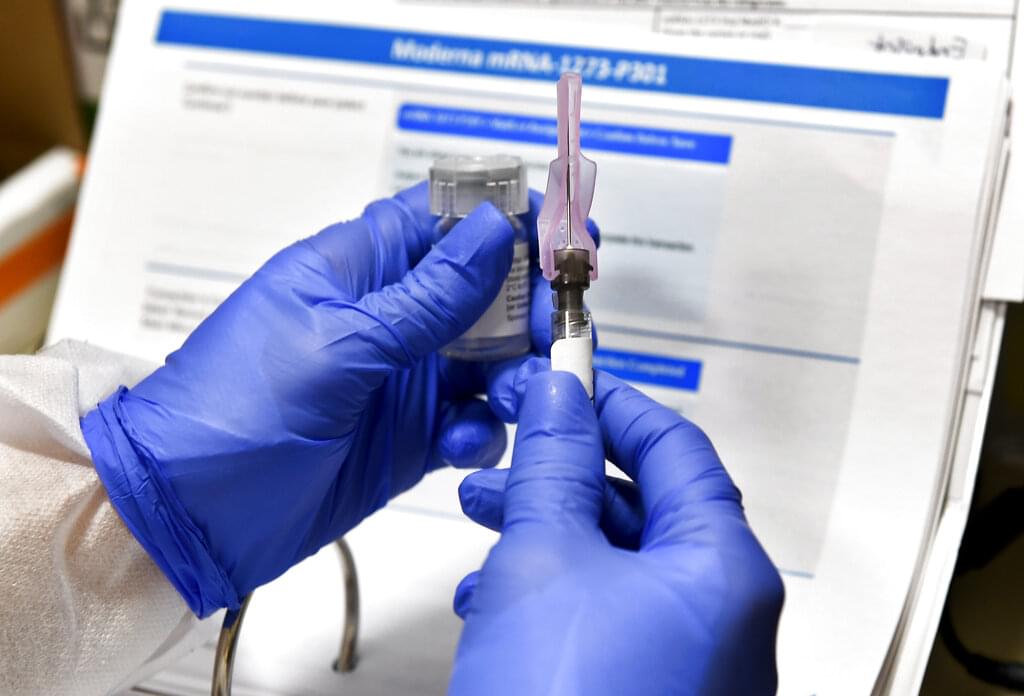
FILE - In this July 27, 2020, file photo, nurse Kathe Olmstead prepares a shot that is part of a possible COVID-19 vaccine, developed by the National Institutes of Health and Moderna Inc., in Binghamton, N.Y. Moderna Inc. says it will ask U.S. and European regulators to allow emergency use of its COVID-19 vaccine as new study results confirm the shots offer strong protection. (AP Photo/Hans Pennink, File)